You can also find our publications on:
https://zenodo.org/communities/miel-doctoral-network
Conceptual Modular Design for Continuous Pharmaceutical Processes: A Case Study on Ibuprofen
Tuse Asrav, M. Alvarado-Morales, G. Sin
Modular process design offers product, volume, and location flexibility to meet the dynamic needs of the pharmaceutical industry. This study highlights volume flexibility by utilizing small-scale process units that can be scaled efficiently by numbering up or down to adapt to different production scales in response to uncertain market demands. Through an ibuprofen manufacturing case study, the modular design’s series reactor configuration with bypassing capability is shown to enable rapid capacity adjustments, optimize resource use, and minimize downtime. Unlike conventional plants, modular design demonstrates less sensitivity, greater adaptability, and economic efficiency across varying production scales, offering an innovative pathway for future pharmaceutical manufacturing.
Enabling electrochemical, decarboxylative C(sp2)–C(sp3) cross-coupling for parallel medicinal chemistry
Bingqing Tang, Jennifer Morvan, Pavel Ryabchuk, Evelien Renders, Stefaan Last, Lars Van Eynde, Karolina Bartkowiak, Peter J.J.A. Buijnsters, Alexander X. Jones, Mary-Ambre Carvalho, Justin B. Diccianni
Herein we report the development of an automated protocol for coupling aliphatic carboxylic acids and aryl halides under mild, electrochemical conditions. Carboxylic acids are one of the largest pools of commercially available building blocks utilized in parallel medicinal chemistry to expand structure-activity relationships. However, their usage in decarboxylative cross-coupling reactions to forge C(sp2)–C(sp3) bonds is low due to challenges associated with direct decarboxylation. Redox-active esters (RAE) are commonly employed to increase the reactivity of carboxylic acids for decarboxylative cross-coupling reactions. Previously, coupling reagent byproducts from in situ generated RAEs proved detrimental to transition metal catalysis. We have developed a purification-free protocol for activating carboxylic acids as N-hydroxyphthalimide (NHPI) esters, which are employed in electrochemical decarboxylative cross-coupling in a high-throughput, automated fashion. This automated workflow enables the preparation of compound libraries including PROTACs. By enabling the pool of commercial aliphatic carboxylic acids to be rapidly incorporated into drug-like molecules, this protocol can potentially impact how C(sp2)–C(sp3) cross-coupling reactions are performed in drug discovery campaigns.
Rapid Methylation of Aryl Bromides Using Air-Stable DABCO-Bis(Trimethylaluminum) via Nickel Metallaphotoredox Catalysis
Morgan Regnier, Jonas Djossou, Dr. Andrea Aloia, Prof. Dr. Luca Capaldo, Demi D. Snabilié,, Jasper H.A. Schuurmans, Prof. Dr. Antonio Monopoli, Prof. Dr. Bas de Bruin, Prof. Dr. ing. Timothy Noël
We report a metallaphotocatalytic strategy for the selective methylation of (hetero)aryl bromides via nickel-catalyzed cross-coupling with bis(trimethylaluminum)-1,4-diazabicyclo[2.2.2]octane (DABAl-Me₃), as a commercially available, air-stable, and non-pyrophoric aluminum-based reagent. The method enables a fast, robust, and scalable methylation protocol that broadly accommodates various functional groups while preventing protodehalogenation. Mechanistic studies confirm the unprecedented generation of methyl radicals from an organo-aluminum precursor under photoredox conditions, bypassing the limitations of conventional two-electron pathways. This work expands the toolbox of practical radical precursors and provides a streamlined approach for selective C(sp2)─CH3 bond formation.
Public report: Continuum-scale flow cell model published including open-source code
Lourenço Côrte Vieira
Contrary to most traditional methods, electrochemical synthesis provides a greener, safer and more atom efficient alternative to reaction pathways towards useful compounds In the field of organic synthesis, chemists are now resorting to electrochemistry to achieve novel reactivities and enhanced selectivity, thus avoiding toxic chemicals via potential-induced electron transfer instead 2. However, main reaction pathways are not always fully known, calling for dynamic electroanalytical techniques such as Cyclic Voltammetry (CV) to assess the current-potential curves that evidence the kinetics and mechanism involved 3. Moreover, dealing with complex processes such as multi-electron transfer with coupled homogeneous chemical reactions may
require computational modelling to comprehend and interpret competing interactions as they relate to mass transport, whether in batch or in flow.
Flow Electroreductive Nickel-Catalyzed Cyclopropanation of Alkenes Using gem-Dichloroalkanes
Morgan Regnier, Clara Vega, Dimitris I. Ioannou, Zhenyu Zhang, Timothy Noël
Cyclopropanes are valuable motifs in organic synthesis, widely featured in pharmaceuticals and functional materials. Herein, we report an Efficient electrochemical methodology for the cyclopropanation of alkenes, leveraging a nickel-catalyzed process in continuous-flow. The developed protocol demonstrates broad substrate scope, accommodating both electron-rich and electron-poor alkenes with high functional group tolerance. Beyond dichloromethane as a feedstock methylene source, the methodology enables the synthesis of methylated, deuterated, and chloro-substituted cyclopropanes. Mechanistic investigations suggest the electrogeneration of a nickel carbene as key intermediate. Notably, the reaction operates under ambient conditions, tolerates air and moisture, and achieves scalability through continuous-flow technology, offering a straightforward route to multi-gram quantities with enhanced throughput.
Public report: Method/process for the fluorination / trifluoromethylation of organic modules
Morgan Regnier
Fluorination reactions are a way for synthetic chemists to explore the structure-activity relationship of a given compound as C-H and C-F bonds are sterically similar but electronically different, which gives rise to a different bio-availability of the molecule. Moreover, the integration of 18F isotopes in the target compounds represents an interesting labelling agent to probe the activity of the synthetized active ingredient. As fluorinated compounds are of high added-value, adding a scalable and more sustainable path to achieve fluorination to the toolbox of the organic chemist represents a goal of interest. To this day, a number of methods still rely on nucleophilic or electrophilic sources of fluorine in the form of reagents (e.g Selectfluor ®, NFSI) which can be costly or limit the overall atom efficiency of the process. Recent advances in the field of photochemically driven fluorinations has allowed for milder activation methods while expanding the scope of substrates amenable. More recently, with the advent of electrochemistry as an alternative activation method replacing chemical redox additives, new fluorination methods emerged.
Additionally, as electrochemistry is an inherently heterogenous process, it benefits from a highly efficient mixing. Therefore, the implementation of electrochemical activations in continuous flow setups is of interest to develop efficient and scalable processes. The purpose of this deliverable is therefore to answer the following question: how can an electrochemical fluorination method be designed as an atom economical, safe, scalable and robust process in continuous flow?
Electrochemical C−O and C−N Arylation using Alternating Polarity in flow for Compound Libraries
Jennifer Morvan, Koen P. L. Kuijpers, Dayne Fanfair, Bingqing Tang, Karolina Bartkowiak, Lars van Eynde, Evelien Renders, Jesus Alcazar, Peter J. J. A. Buijnsters, Mary-Ambre Carvalho, Alexander X. Jones
Etherification and amination of aryl halide scaffolds are commonly used reactions in parallel medicinal chemistry to rapidly scan structure–activity relationships with abundant building blocks. Electrochemical methods for aryl etherification and amination demonstrate broad functional group tolerance and extended nucleophile scope compared to traditional methods. Nevertheless, there is a need for robust and scale-transferable workflows for electrochemical compound Library synthesis. Herein we describe a platform for automated electrochemical synthesis of C X arylation (X=NH, OH) in flow to access compound libraries. A comprehensive Design of Experiment (DoE) study identifies an optimal protocol which generates high yields across>30 aryl halide scaffolds, diverse amines (including electron-deficient sulfonamides, sulfoximines, amides, and anilines) and alcohols (including serine residues within peptides). Reaction sequences are automated on commercially available equipment to generate libraries of anilines and aryl ethers. The unprecedented application of potentiostatic alternating polarity in flow is essential to avoid accumulating
electrode passivation. Moreover, it enables reactions to be performed in air, without supporting electrolyte and with high reproducibility over consecutive runs. Our method represents a powerful means to rapidly generate nucleophile independent C X arylation compound libraries using flow electrochemistry.
Measurement of Temperature in Parallel Channel of Microfluidic Reactors
Abdullah Khan, Morgan Regnier, Dušan Kopecky
Measurement of temperature in microfluidic devices during their operation is a common and important task. Knowledge of the temperature value, steepness of the temperature gradient, or temperature evolution over time is crucial for subsequent modelling or control of chemical processes taking place inside the device. However, due to spatial limitations, the integration of sensors can be very challenging. Herein, the development of a temperature monitoring system integrated into a flexible polydimethylsiloxane (PDMS) based microfluidic reactor is presented for use in electrochemical flow cells. The system is based on a set of thermistors mounted in one of the parallel channels of the multichannel microfluidic reactor. Sacrificing one of the channels allows the integration of inexpensive and relatively large sensors into the device and the measurement of the temperature directly inside the electrochemical system even under harsh conditions. The static
and dynamic properties of the system are evaluated. Furthermore, the fabricated system can be easily integrated into the standard manufacturing process of any microfluidic device.
Enhancing electrochemical reactions in organic synthesis: the impact of flow chemistry
Morgan Regnier, Clara Vega, Dimitris I. Ioannou and Timothy Noel
Utilizing electrons directly offers significant potential for advancing organic synthesis by facilitating novel reactivity and enhancing selectivity under mild conditions. As a result, an increasing number of organic chemists are exploring electrosynthesis. However, the efficacy of electrochemical transformations depends critically on the design of the electrochemical cell. Batch cells often suffer from limitations such as large inter-electrode distances and poor mass transfer, making flow cells a promising alternative. Implementing flow cells, however, requires a foundational understanding of microreactor technology. In this review, we briefly outline the applications of flow electrosynthesis before providing a comprehensive examination of existing flow reactor technologies. Our goal is to equip organic chemists with the insights needed to tailor their electrochemical flow cells to meet specific reactivity requirements effectively. We also highlight the application of reactor designs in scaling up electrochemical processes and integrating high-throughput experimentation and automation. These advancements not only enhance the potential of flow electrosynthesis for the synthetic community but also hold promise for both academia and industry.
Electrosynthesis of Aryliminophosphoranes in Continuous Flow
Rodrigo Costa e Silva, Clara Vega, Morgan Regnier, Luca Capaldo, Lars Wesenberg, Grace Lowe, Kleber Thiago de Oliveira, Timothy Noël
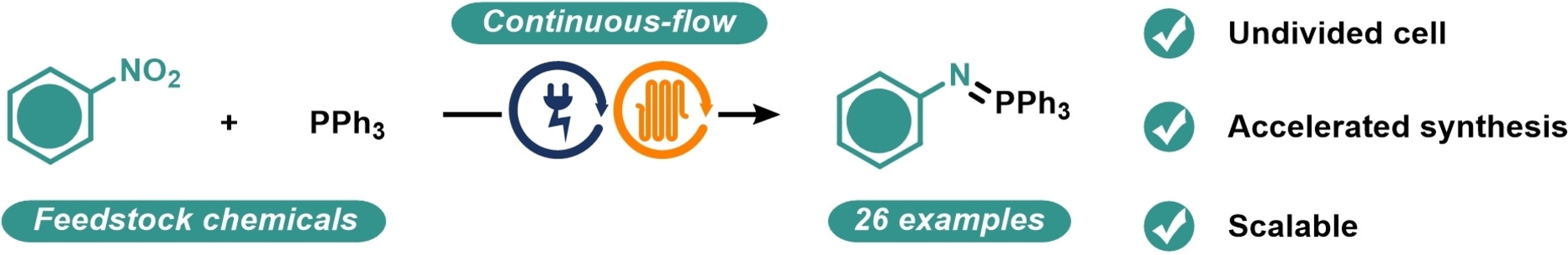 A practical electrochemical method for synthesizing aryliminophosphoranes from widely available nitro(hetero)arenes in a continuous-flow system is presented. The utilization of flow technology offers several advantages to our approach, including the elimination of the need for a supporting electrolyte and enhanced scalability. Our method demonstrates good tolerance towards various functional groups, with electron-deficient nitroarenes being particularly suitable for this strategy. In addition, we have demonstrated the versatility of aryliminophosphoranes as intermediates in synthesizing anilines, amines, and amides. To further enhance the utility of our approach, we have developed a telescoped method that utilizes a tube-in-tube setup for the in-situ production of isocyanates.
A practical electrochemical method for synthesizing aryliminophosphoranes from widely available nitro(hetero)arenes in a continuous-flow system is presented. The utilization of flow technology offers several advantages to our approach, including the elimination of the need for a supporting electrolyte and enhanced scalability. Our method demonstrates good tolerance towards various functional groups, with electron-deficient nitroarenes being particularly suitable for this strategy. In addition, we have demonstrated the versatility of aryliminophosphoranes as intermediates in synthesizing anilines, amines, and amides. To further enhance the utility of our approach, we have developed a telescoped method that utilizes a tube-in-tube setup for the in-situ production of isocyanates.
Press release - 30th May 2023
Sustainable syntheses for the production of fine chemicals
The international research network „MiEl“ aims to develop novel synthesis technologies that use renewable and sustainable electricity for the production of pharmaceuticals and other valuable chemicals.
Electrochemical synthesis pathways are an efficient use of renewable energies. In the research network „MiEl“ (Grant Agreement No. 101073003), led by the Fraunhofer Institute for Chemical Tech-nology ICT and funded by the Marie Sklodowska-Curie Program of the European Union, 12 international PhD students will develop electrochemical synthesis processes for mass production, applying concepts of microfluidic cells. To make the syntheses efficient and powerful, simulations and modelling will accompany the system development. The technologies will be developed for industrial applications in collaboration with relevant industry.
The special feature of this type of project is that the doctoral students will have the opportunity to be trained by an interdisciplinary European consortium of industrial companies and research organizations. The PhD students recruited by the industrial partners will complete research stays at the academic partners, while the PhD students at the academic institutions will complete secondments mainly in industry. All 12 doctoral candidates are linked together in a scientific program to solve interdisciplinary research questions in the very promising field of electrosynthesis. This creates a valuable network where academic know-how can be quickly transferred into applications for industry or society. The doctoral students will receive excellent training in both scientific, technical and soft skills like management or presentation techniques. Their new knowledge of research and development methods will be trained in practice applying the newly developed models, materials, and processes to develop new synthetic routes for fine chemicals and pharmaceuticals.
Contact
Dr. Robin Kunkel (Project Coordinator)
+49 721 4640 504
Carolyn Fisher
+49 721 4640 277
Fraunhofer Institute for Chemical Technology
Joseph-von-Fraunhoferstraße 7
76327 Pfinztal (Berghausen), Germany
Funding

Funded by the European Union (Grant Agreement no. 101073003). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the granting authority can be held responsible for them.
Social media
LinkedIn: MiEl – Doctoral network for microprocess engineering for electrosynthesis
Twitter: project_MiEl
Data privacy information
Imprint and Disclaimer
